Hello!
I am wondering if someone here can help me with doing my own FEC test. I have a microscope and purchased the Eggzamin McMaster Method slides (https://www.eggzamin.com/eggzamin-products/eggzamin-fec-accessory-kit) but my results seem much higher than I would think.
I have two mares who are very healthy and shiny, are out pretty much 24/7 on a lot of land (15 acres-ish). I dewormed in April with Quest, and then this fall have tried an herbal dewormer as they have historically been low-moderate shedders. I thought I’d test to see if it was working. In November, I sent off manure for FECs and got the results of 18 EPG for Mare 1 and 74 EPG for Mare 2. After doing the herbal dewormer I wanted to see if these dropped or if I should hit them with a fall dewormer now that we’ve had solid frosts (I’m in VA).
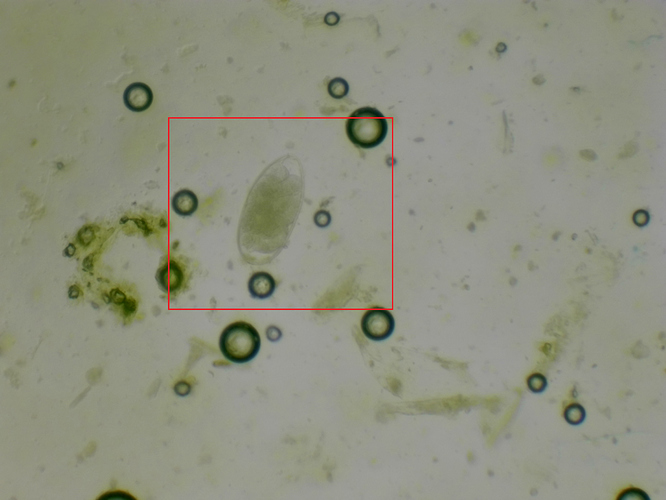
I did the test yesterday, and I believe I followed all instructions correctly. I measured the manure - 4 grams on a kitchen scale (yes, my husband hates me) - with 26 ml of the solution. Filled both chambers and counted 40 eggs for Mare 1 and 83 for Mare 2. This is a count of all eggs within the grid pattern on each side of the slide (combined). The instructions say to multiply that by 25. This would give me 1000-2000 EPG. I feel like I am definitely doing something wrong but I’m not sure what!


 No study has shown they do, and several have shown the stuff tested didn’t.
No study has shown they do, and several have shown the stuff tested didn’t.
