Great news. Hope it helps tomorrow
@RAyers. Hi! Thanks for the rapid response. Yes, I had read the FDA notice and the required contraindications. For what it’s worth, it seems hard/impossible to learn product limitations or restrictions on the Q30 web site, other than a discussion about fitment.
FDA release says the device can be worn up to 4 hours. But, it does not say what the “relaxation” / off time required between 4 hour stints. The research on intraocular pressure mentions the pressure remains for a time after the vein restriction is removed. That makes sense.
The other FDA release notes say do not use it in the following conditions:
- must be 13 yrs or older to use
- Increased pressure in the skull (including uncontrolled ocular-glaucoma)
- Increased presence of acid in the body or excessive blood alkalinity
- Open head injury (including in or around the eye) within the past six months
- Pseudotumor cerebri (false brain tumor)
- Presence of brain or spinal shunt
- Accumulation of cerebrospinal fluid within the brain
- Known seizure disorder
- Known trachea abnormality
- Known airway obstruction
- Known carotid hypersensitivity
- Blood clot in the brain
- Increased likelihood of blood clotting (coagulation)
- Collections of small blood vessels in the brain that are enlarged and irregular in structure
- Skin injury, rash, or other abnormality on or around the neck
Maybe I’ve missed it, but can’t find the product use limitations listed on the Q30 site. Usually for a retail / consumer product which is sold directly this info is available, before purchase. There is a link to the FDA release but, one needs to dig in News to find it. Most consumers aren’t going to look that far. They assume since it’s approved for consumers that it’s approved. When you move to purchase it, they indicated it’s for 13 and older.
I’m not trying to nit pick. I would like to learn what it does, how it does it, and what the restrictions are on its use.
As an aside, I am curious about its use in sports car racing where attenuation of impact shock is extremely important. There is no info on that application. That’s fine. Just curious.
Let me start this by saying I have postural hypotension, some days worse than others.
I wore my Q30 collar around the house, in air-conditioning, this morning. Since I always get vertigo if I lean back and do not keep my face vertical I tested that out, I could sit, lean way back, and I did not get vertigo even though I kept my skull in line with my backbone!
After an hour my body wanted it off, fine. For the next two hours my postural hypotension is back, and I feel somewhat dizzier without the collar than I did wearing the collar and I am “furniture walking” and “wall walking” more. I guess I am pretty much back to my “normal”.
I will see how I do with my lesson tomorrow. I REALLY NEED to explain to this lesson horse that he does not need to turn his spine and back into a bar of concrete when I ride him. At 30 minutes a week this may take months, but at least now I will be able to start the process.
Many months ago I had convinced him to relax under me (this took me around 3 months), then he got partially leased to another rider and his spine is STIFF again and he does not want to relax it for anybody.
Generally, as with any medical device or drug, the limitations are listed in the Instructions for Use (IFU). I never see J&J list the limitations for their pacemakers or hip replacements on the website.
Pacemakers and hip prostheses aren’t marketed directly to the lay public, though. If someone is getting a pacemaker or artificial hip, they have been evaluated by a physician who has determined they’re a suitable candidate.
I’m certainly intrigued, but it seems like the sort of thing that is likely to have potential downsides if it works well enough to have the intended effect.
Even the doctors rarely see the IFUs as they are the package inserts. The only time the doctor sees that would possibly be in the OR.
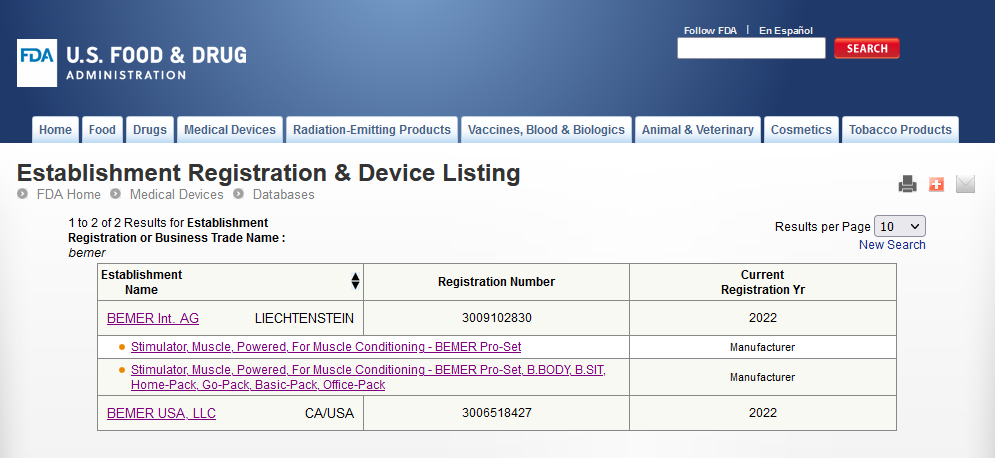
My point is that even Class I medical devices (although I believe this device is Class II due to FDA pre and post market surveillance requirements), don’t ever post contraindications online, e.g. contact lenses, tongue depressors, exam gloves, syringes,… Look at BEMER products. There is a reason they were banned from human use and pulled from the market by the FDA. But their website never mentions that.
For those of us who are apparently living under a rock…could you tell us more?
My point is, regardless of what information is on the website or package insert, before someone gets a new hip prosthesis they have to see a physician who’s responsible for knowing the indications and contraindications for that device and determining whether the patient is a suitable candidate.
When it’s marketed directly to the lay public, there’s nobody there to make sure it’s used appropriately and fitted correctly, so it had best be something that’s quite idiot proof and safe even when not used exactly as directed.
I’d love to get a take from a neurologist or neurosurgeon not involved in the development or marketing of the collar.
Ummmmm what? That sure as heck is news to me in the land of “Why is everyone and their cat buying a BEMER set?”
If you look at the FDA MAUDE database where mandatory device related serious injury or patient death is reported you will find BEMER human products. This resulted in a mandatory product recall of BEMER products from the human market.
Off the top of my head this was 2 years ago.
This? But it certainly doesn’t appear to be an all out ban on these products for people. The Bemer site is still marketing human equipment.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=185325
Also, out of seven reports of adverse events, four relate to implantable neurostimulators, one an insulin pump. One guy thinks it cause prostate cancer. And there’s this gem:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/detail.cfm?mdrfoi__id=9641774&pc=ISA
This definitely doesn’t look banned?
Our laws allow for direct marketing to consumers. Orthopedic devices are marketed to the consumer, doctors. They, many times never are truly educated on the indications or contraindications for a device. That is how I earned a large chunk of income perusing orthopedic device product liability in the US.
I agree about the idea of “idiot proof” but again, let’s look at BEMER products, shock wave machines, TENS devices, lasers,…
Thank you for this discussion.
@Sticky_Situation I agree. Direct consumer marketed devices are a different kettle of fish, IMHO. I always hope that the manufacturer has made a “fail safe” product, meaning if it is used outside of the design envelope, that it does no harm, it merely doesn’t work. But, these days it’s on us to check that out and not assume they are so professional. A consumer product will always be used beyond the intended application, either through ignorance or a cavalier attitude (it didn’t say not to use it that way). Product liability lawyers try to protect the company by publishing the warnings (often in micro-fine print) so they can say to potential litigants that they were warned. Other than the 13 year old limit, there are no limits published (online) by the company. (I couldn’t see how to download the product manual were the constraints may be listed.) Only the FDA notice is available. But that’s a subset of the constraints.
The FDA revealed 4 hour consecutive use limit is interesting, given there’s no “off time” required or mentioned. Can it be 1 minute? (That’s perhaps non-sensical.) 1 hour off time? 2 hours? They clearly don’t want this excess blood in the brain for arbitrary lengths of time.
So, the professional competitor / trainer at our barn routinely has many consecutive training rides back-to-back with grooms tacking up the horses to be ready. It takes a long time, certainly longer than the 4 hour limit. Sometimes a lesson is taught interspersed with the training rides. The helmet may go on and off at that time, or she may hop on the student’s horse sometimes. Is it an acceptable rule to always wear the device if you have your helmet on, because if your helmet is on you’re likely to be mounted, shortly? I can’t tell.
Anyway, it’s a good and helpful conversation everyone!
I got to wear my Q30 collar today for my riding lesson. This is the horse with the jackhammer on concrete sitting trot.
I put on the collar right before I mounted. I felt it around my neck at first and then I quickly forgot it was there. As well as my MIPS helmet I had my Columbia cooling neck gaiter on up over my head, my cooling collar fan just a little bit lower on my neck, and my ice vest on my torso. The Q30 collar did not interfere with any of these things and I just forgot it was there.
I felt “braver” and a little bit more competent riding. I held onto my grab strap and leaned way back keeping my head back (which before caused vertigo) and I had no dizziness.
The difference for the sitting trot was amazing. Instead of my brain going “no, No, NO, a thousand times NO!!!” after two strides I did three or four sitting trots going maybe a quarter of the way around the ring. My brain did not protest this at all.
However my back was not happy with me. I asked MJ for slow trots and I aided for slow trots and he mostly obeyed me for this. A few strides were nice and soft but for the rest the jackhammer on concrete started coming back. Debbie got after me for slouching, and when I corrected my slouching my back started screaming at me especially between my shoulders, but my brain was fine. She also got after me for not softening my rein enough when we started our first trot though MJ made none of his usual sort of emphatic protests (he does not cuss me out, he just mutters under his breath, today I did not “hear” the muttering though he showed Debbie that he was not totally comfortable with my hands at the sitting trot.) My neck did not hurt at all. I improved for the other sitting trots. MJ’s back was not as horribly stiff as before but I still had to absorb a lot of motion with my back.
Our transitions were smoother than usual and I could use lighter aids.
Debbie’s next lesson was coming into the ring to warm up, so MJ and I had to work around the other horses.
I SAT THE TROT AGAIN! without any major problems.
I took the Q30 collar off right after I dismounted. I felt a little bit more dizzy walking back to the barn, just about normal for me after riding in the extremely humid air. I do notice a difference between walking with the Q30 collar on and when I do not have it on. Without it my sense of balance is worse and I have to take more care when walking to keep my balance.
So now I will be able to work on MJ’s sitting trot. This is good. As he regains his faith that I will not go BAM, BAM, BAM on his back he should relax it more. Every once in a while during the sitting trot I got a few strides that were pretty soft for MJ, and I am eagerly expecting that I will be able to get him to relax into a decent, not-very-jarring, sitting trot.
So far I am pleased with the collar.
Sounds good Jackie. Hope your back toughens up 
Next morning–I feel fine, my spine and back do not hurt at all and I am walking fine.
I was expecting to be sore this morning. Nope. My gripping muscles feel fine, my butt feels fine, and my neck feels fine too.
Simply amazing. I was ready to be hurting all over. I did wear my BOT back brace and neck dickie last night but that has never prevented my morning-after-my-lesson aches and pains.
Sounds like things are going well